Introduction to RKKP
The Danish Clinical Quality Program– National Clinical Registries (RKKP) constitutes the infrastructure of the Danish clinical quality registries and the Danish Multidisciplinary Cancer Groups (DMCG).
RKKP’s primary objective is to ensure a continued improvement in the utilization of the registries in a clinical as well as managerial, and research oriented sense.
Objectives and results
RKKP’s primary objective is to ensure a continued improvement in the utilization of the Danish clinical registries in a clinical as well as managerial-, and research oriented sense. The programme has since its introduction focused on preserving the framework for prioritization and conduct of the databases; improving the use of existing data; standardization of in-, and outputs in relation to the databases as well as product- and methodological development.
RKKP designs new clinical registries, and facilitates re-use of available data in hospitals and GP-surgeries to lighten the data collection burden, and optimize the use of available data sources. The formation of a registry can be initiated either top-down – RKKP asking clinicians to collect data on a given topic – or bottom-up with clinicians asking RKKP to manage a database on a topic of their interest. The relevance of conducting a database on a given topic is decided on by weighing; disease severity, incidence/ prevalence, quality problems/ possibility for improvement, resources and appropriateness, political and patient preferences and so on.
RKKP manages about 85 clinical registries, containing information about individual patients, used for improvement of quality, research and surveillance purposes. The programme is exempt from patient consent to data-collection. A catalogue of all the clinical databases containing annual reports can be found here
Through development and reporting of quality-indicators and -standards for good clinical practice this work is facilitated in favour of improving the overall quality of patient treatment in the Danish hospitals and medical practices. The process can be visualized as a system of PDSA circles;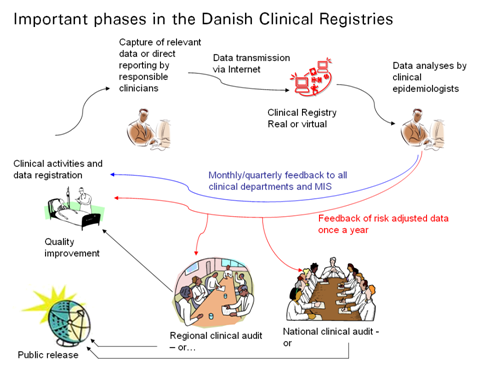
Data is registered by clinical personnel, gathered and reported through data-collection systems. Thereupon accumulated and analysed by epidemiologists, to be discussed and concluded on at regional and national audits. At these audits clinicians determine indicators and standards for good clinical quality, and recommendations are reported back to clinical personnel for quality improvement, and released in public for transparency and accountability, and potentially further scrutiny.
In favour of improving quality and ensuring accountability RKKP is obliged to supply feedback to clinicians and management information systems monthly/quarterly, and produce a yearly report with analysis and recommendations per database.
Organisation
The Clinical Registries in Denmark are founded on a national initiative, mandated by law and regulated by national government, but financed and owned by regional governments. Each registry has to pass appraisal in the National Health Authority every three years, where it is assessed whether it fulfils national criteria for functionality, data safety and methodology.
RKKP was constituted in September 2010 by a fusion of 4 existing support organisations. It has a yearly budget of app. 8 million Euro.
RKKP consists of:
- a center responsible for analytical methods including defining relevant register population, indicator algorithms, risk adjustment, relevant interpretation of results in the yearly reports, online feedback and communication with and support to the regions and clinical departments with regard to use of data for quality improvement.
- professional boards appointed by professional medical and nursing societies for each clinical registry, representing the main clinical stakeholders.
For more information please contact rkkp@rkkp.dk
